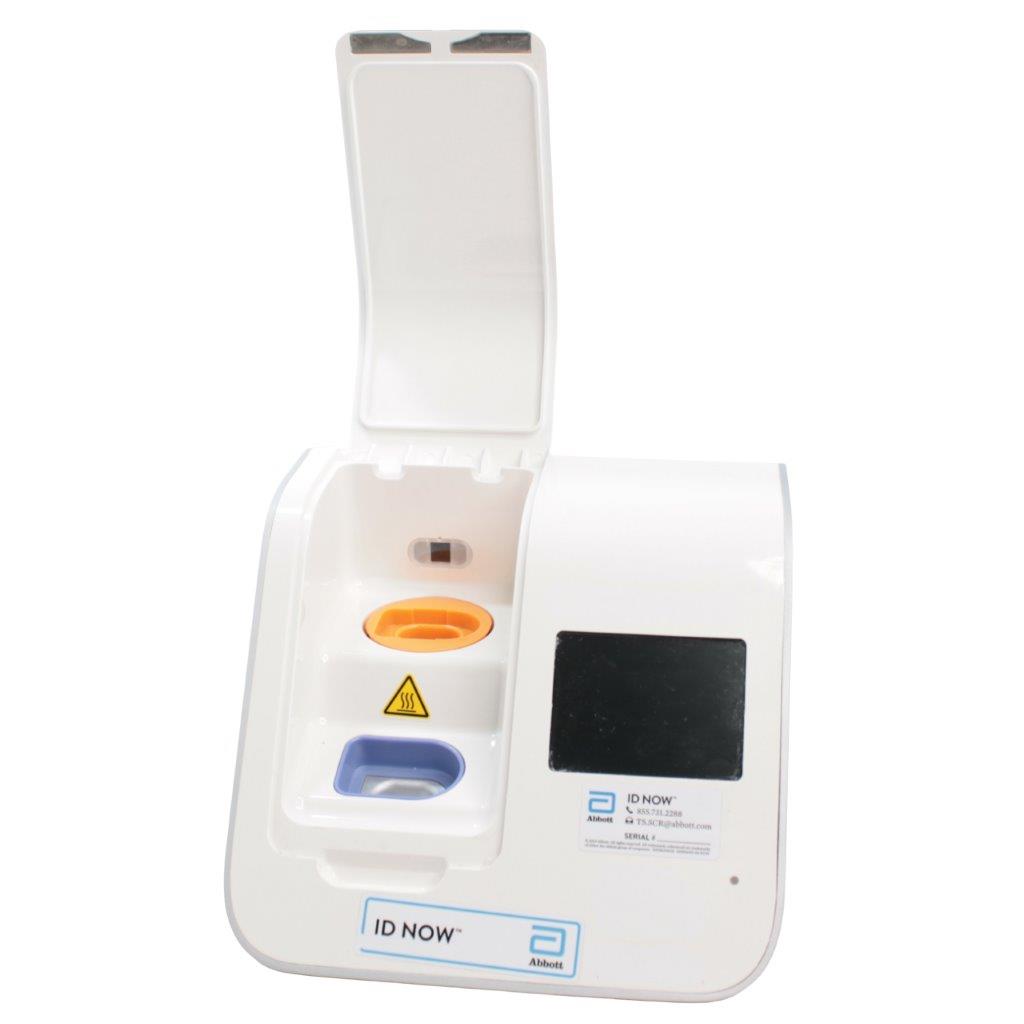
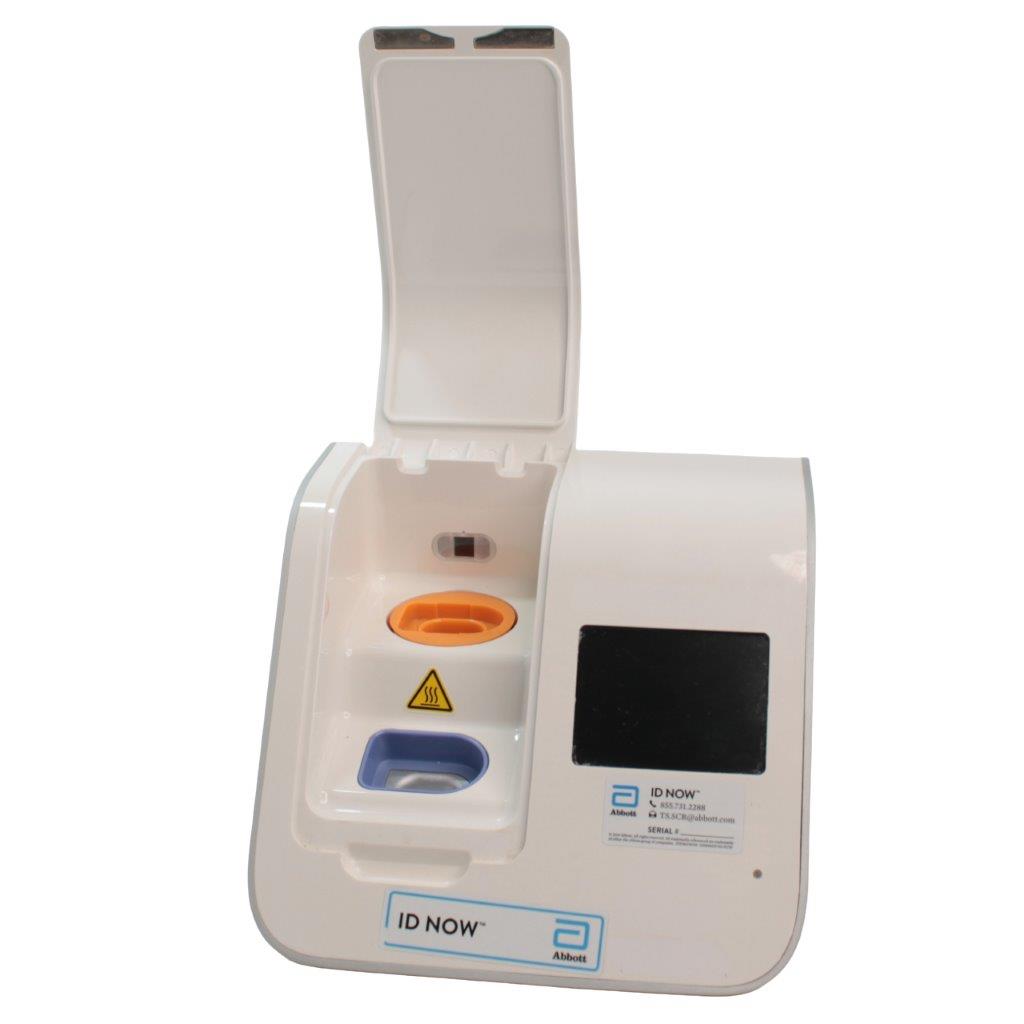
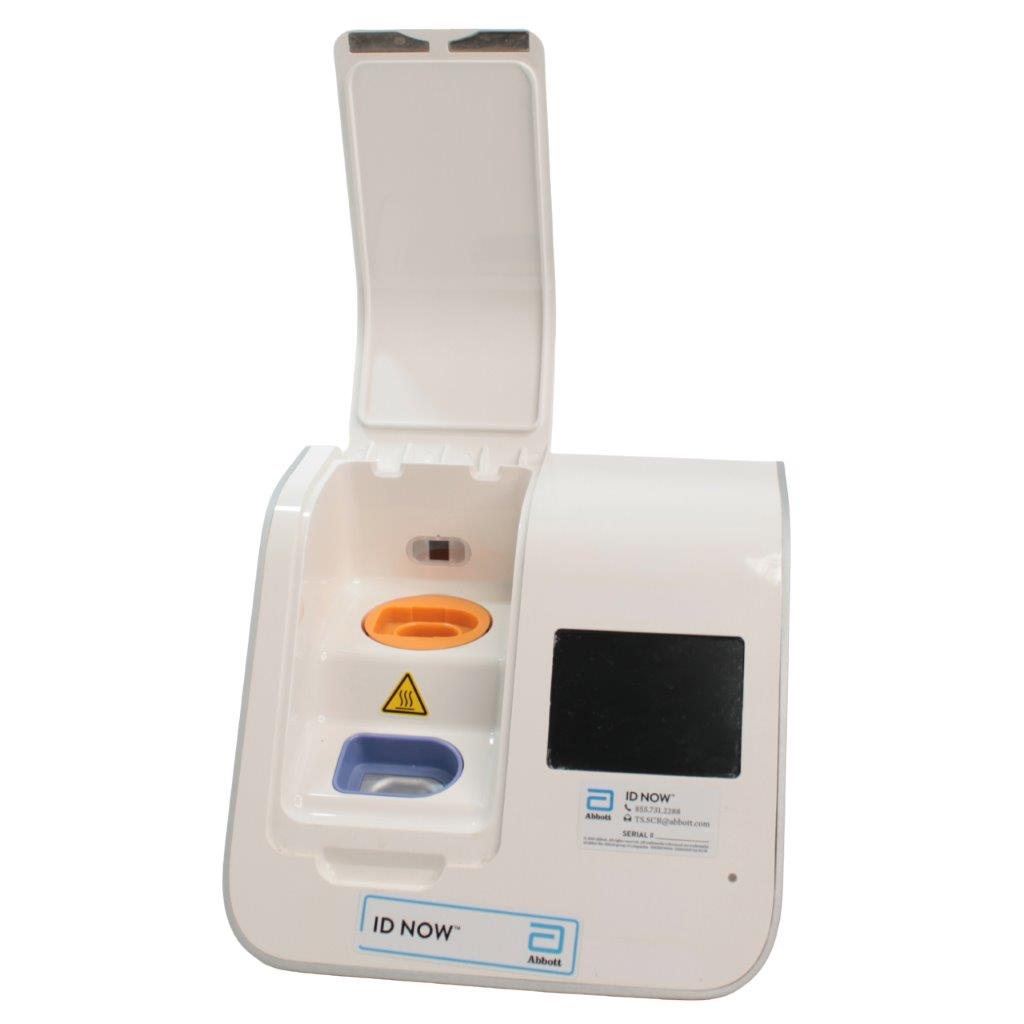
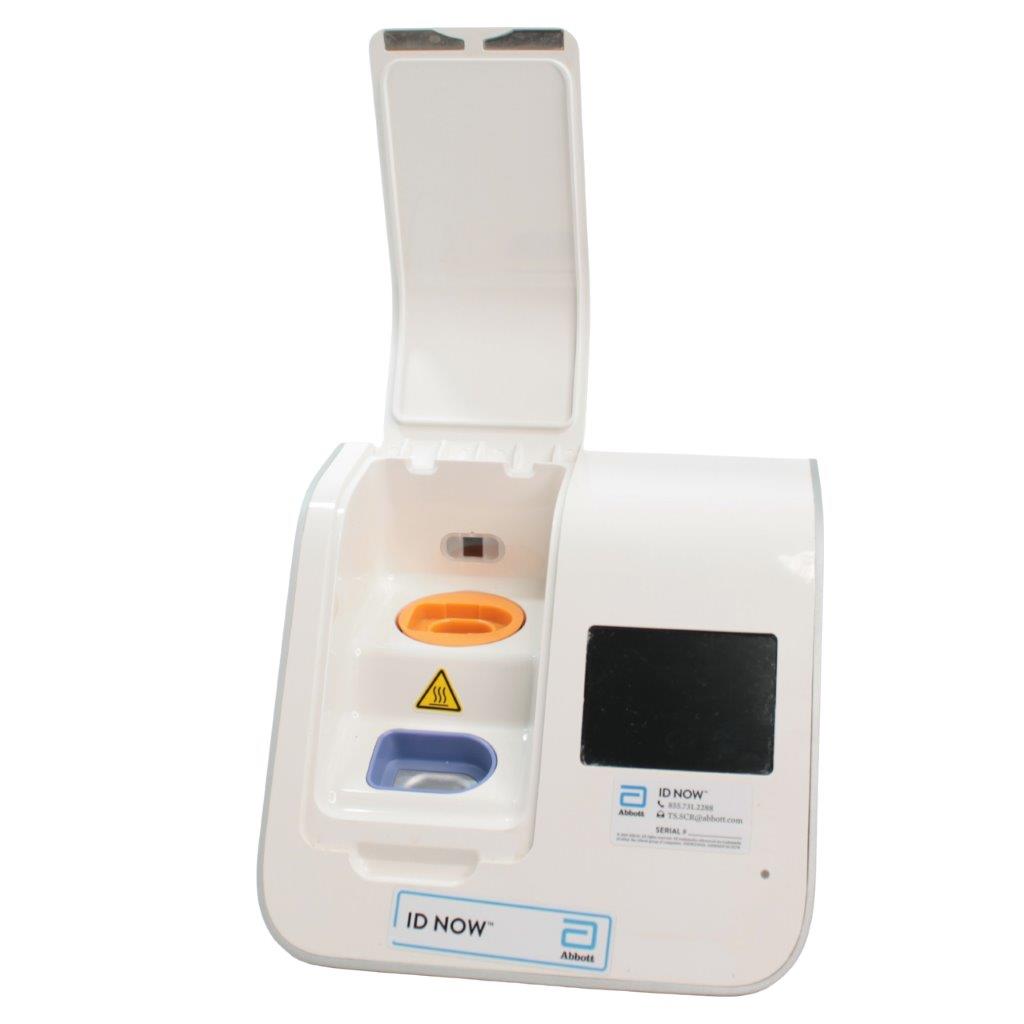
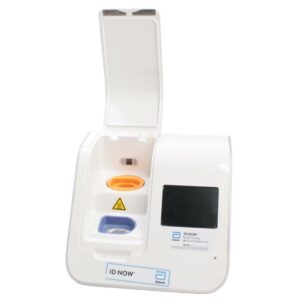
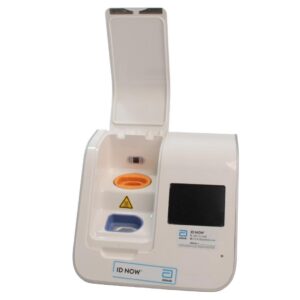
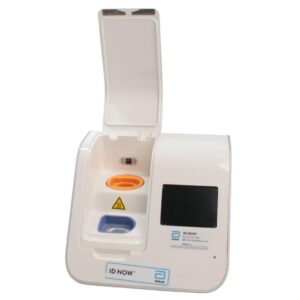
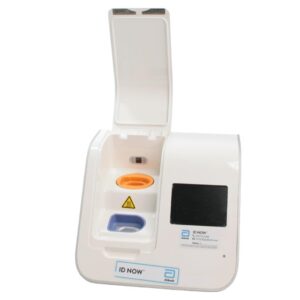
Abbott ID NOW EQ002001S Rapid Molecular Diagnostic Test Instrument
$200.00
We might not have it
Abbott ID NOW EQ002001S Rapid Molecular Diagnostic Test Instrument
Condition: Used, Good Condition, powers up, see pictures with menu
The Abbott ID NOW EQ002001S is a molecular diagnostic device designed for rapid detection of infectious diseases. Known for its portability and speed, the ID NOW system utilizes isothermal nucleic acid amplification technology, delivering molecular results in minutes. This device is frequently used in healthcare environments, especially for point-of-care testing in hospitals, clinics, and laboratories.
Key Specifications:
- Model Number: EQ002001S
- Manufacturer: Abbott
- Type: Molecular Test Instrument
- Test Types: Supports a range of molecular tests for infectious diseases, including Influenza A & B, RSV, and Strep A.
- Condition: Used, generally in working condition, with a power supply unit (PSU) included.
- Ports:
- 2 USB Type-A ports (likely for external peripherals like keyboards or USB drives)
- LAN Connector (used for network connection or data transmission)
- Power Supply Unit (PSU): Included with the device, ensuring it is operational without needing additional accessories.
Features:
- Rapid Molecular Testing: Delivers fast, lab-quality results at the point of care. This technology provides results in 5 to 15 minutes depending on the test.
- Portable Design: Compact and lightweight, the ID NOW system is easy to transport and set up in various locations, ideal for high-demand settings.
- Multiple Connectivity Options: The device features 2 USB Type-A ports and a LAN connector for added flexibility in data transfer and device connectivity.
- Wide Application Range: Used primarily for testing common infectious diseases, with a focus on respiratory illnesses like influenza, and RSV.
- Ease of Use: The system is designed for point-of-care testing with minimal training required, making it a highly efficient solution for clinicians.
Applications:
- Hospitals & Clinics: Ideal for rapid testing in emergency rooms, urgent care centers, and clinics.
- Mobile Testing Units: Because of its compact form factor, the Abbott ID NOW system is also suitable for use in mobile testing centers or temporary testing locations.
- Pharmacies & Laboratories: Utilized in pharmacies for quick diagnostics, allowing for immediate response to patient needs.
Pros:
- Fast Results: Provides near-instant molecular results, which is crucial for fast decision-making in critical situations.
- Portable & Compact: The small footprint makes it highly versatile and easy to move between different testing locations.
- Multiple Connectivity Options: The presence of USB and LAN ports offers flexible data management and integration with other systems.
- Broad Disease Testing: Compatible with a wide array of test cartridges (e.g., COVID-19, influenza, RSV, etc.), making it a versatile molecular testing platform.
Cons:
- Used Condition: As a used device, performance or wear may vary depending on previous usage. It’s recommended to thoroughly test the device before critical use.
- Limited to ID NOW Test Kits: The device only works with Abbott ID NOW test cartridges, limiting flexibility to a specific line of consumables.
Maintenance & Upkeep:
- Regular Calibration: Molecular test instruments like the Abbott ID NOW require periodic calibration to ensure accurate results.
- Cleaning and Disinfection: Ensure the device is properly cleaned and disinfected after each use, especially in healthcare settings.
Related Items:
- Molecular diagnostic system
- Abbott ID NOW test kits
- Point-of-care testing device
- USB-powered medical device
Legal Disclaimer and Terms of Purchase:
The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If so, do not bid on or purchase this item unless you are an authorized purchaser.
Responsibility of Buyer
By purchasing this item, the buyer assumes full responsibility for the purchase, preparation, inspection, testing, calibration, and use of the item, including ensuring compliance with any applicable regulatory requirements. Medical and dental items must be inspected by a qualified biomedical technician before being placed into use. It is solely the buyer’s responsibility to ensure the item’s safety and suitability for its intended purpose.
Indemnification and Release of Liability
The buyer agrees that the seller shall not be held responsible or liable for any injuries, damages, or losses, whether incidental or consequential, arising from the purchase, preparation, or use of the item. The buyer further agrees to indemnify, defend, and hold the seller harmless from any claims, actions, or demands, including, without limitation, reasonable legal fees, arising from or related to the buyer’s purchase, preparation, or use of the item.
No Warranty
The seller makes no warranties, express or implied, regarding the condition, functionality, or safety of the item, including but not limited to any implied warranties of merchantability or fitness for a particular purpose. The buyer acknowledges that the item is sold “as is” and “with all faults.” The only exception to this is the seller’s 15-day refund guarantee, which applies only to the purchased item and only for 15 days from the shipping date. No other warranties, guarantees, or remedies apply.
Final Agreement
By purchasing this item, the buyer acknowledges and agrees to the terms of this disclaimer, accepting full responsibility for all aspects of the purchase and use of the item.
NL28
| Weight | 5 lbs |
|---|---|
| Dimensions | 10 × 7 × 9 in |
You must be logged in to post a review.














































Reviews
There are no reviews yet.